MMP 2014 Community Questions Report regarding access to clinical trials for rural vs metropolitan patients.
Thank you to everyone that participated in our survey. You were obviously passionate about the ‘rural vs metro access to clinical trials’ topic as 216 of you participated. Your contribution to these surveys and consumer forums is immensely important and helps the Melbourne Melanoma Project (MMP) to keep focussed on what is important to patients.
In summary 94% of the respondents to the survey were patients. Of the people that completed the survey 58% were metropolitan and 48% rural. When asked how valuable clinical trials are with respect to developing new treatments for melanoma 96% said trials were at least ‘somewhat valuable’, with 65% of you saying they were extremely valuable. Four percent were unsure. Ninety-four percent of respondents thought that clinical trials were definitely a ‘valuable’ means of improving survival/treatment outcomes for patients. Six percent were unsure.
When considering patient access to clinical trials in Victoria 24% believed that access was equal no matter where you lived, 26% thought access was not equal and 50% were unsure. Interestingly only 1% of respondents thought Australia invested enough funds into melanoma clinical trials. Forty-five percent said there was not enough investment and 54% were unsure. Twenty-eight people added additional comments, many indicating that there is a lack of clear and concise information about the government’s actual outlay for clinical trials. Others called for more melanoma research to be done.
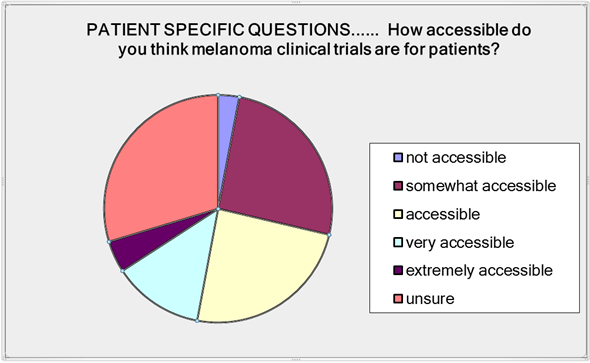
In the patient specific questions we asked about clinical trial accessibility. Three percent said trials were inaccessible; 26% thought they were somewhat accessible; 23% said accessible; 13% very accessible; 4% extremely accessible and 30% were unsure (Figure 1).
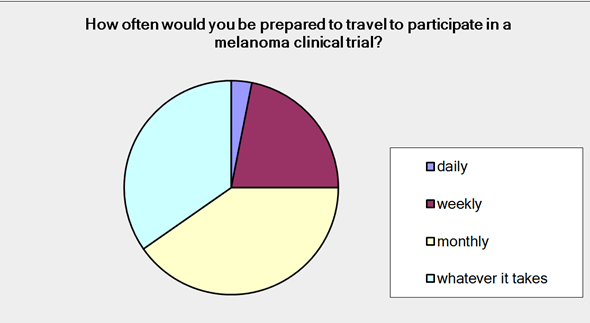
Patients participating in the survey were asked how far they were prepared to travel to be part of a clinical trial. 100% of them responded saying that they would be prepared to travel to varying degrees: 31% 0-1 hours; 51% 1-5 hours; 4% +5 hours; 14% interstate. Ninety-eight percent of the patients surveyed responded to the question ‘How often would you be prepared to travel to participate in a clinical trial?’ Figure 2 shows us that 3% of you considered daily as an option; 22% thought weekly; 40% monthly and 35% would travel as often as necessary.
The final question of the survey asked patients to rate any melanoma clinical trials they had participated in. Thirty-six percent of respondents had never participated in a clinical trial and 37% had not been invited to be part of a clinical trial. Of the 27% that had participated: 1% perceived no benefit; 5% thought it somewhat valuable; 9% described participation as valuable; and 12% rated it very or extremely valuable. Thirty-five patients added further comments to this section. Most indicated that patients were more than happy to participate and therefore help others. Some would like more feedback on trial results others had concerns/questions about eligibility, placebo options, standard treatments and alternative treatments.
So what are the raw take away messages from this survey?
- Melanoma clinical trials are very valued as a means of improving treatment outcomes.
- Victorian patients are very willing to participate in trials even if it means travelling to do so.
- The public are not aware of the Government’s actual level of support for melanoma clinical trials.
- Melanoma patients still have many unanswered questions.
Conclusion
In hindsight some of our survey questions could have been tailored a bit better, (for instance it would be interesting to compare responses from Stage I & II patients directly with Stage III & IV patients) however we feel the survey has been a huge success in that it has generated greater dialogue between all parties in the melanoma community. Some of these results were used in the MMP 2104 Scientific Exchange Meeting. A report on this meeting can also be found on our website.
We thank you again for participating in the Melbourne Melanoma Project and in our survey. You make a difference.
Yours sincerely
The MMP Consumer Reference Group
