Benefits of collaborating with MRV
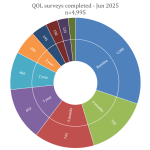
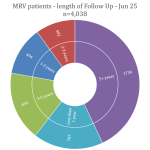
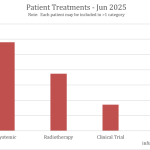
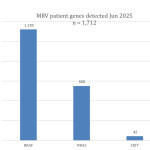
Collaborate now with MRV researchers to access our one-stop-shop cohort resource for melanoma research. Our clinical data and biospecimens have contributed to 80 research projects and generated more than 200 publications. This 12-year-old cohort has an extensive follow-up, as a result of its annual request for data from MRV participant’s clinicians, adding considerable value to your new research questions.
We have assisted local, national and international researchers with their projects, validations and consumer engagement.
If you are interested in health costs, patient-reported outcomes, demographics, validations, biospecimens, and/or clinical data you should explore our resource pages and then contact us for a preliminary estimate of numbers.
Collaborating With MRV – Next Steps
To request a preliminary query regarding the approximate numbers of cases, data or biospecimens for your next project/funding application please contact MRV.
Approval Process
All proposed projects are required to be scientifically reviewed and approved by the MRV Governance Committee prior to receiving any MRV material.
This is done by :
Questions regarding scientific rigour and/or approval generally occur within two weeks.
Conflicted Governance Committee members are not included in the review or decision. External experts can be consulted if required.
Costs
The following are the cost recovery charges for data extractions, biospecimens and a variable administration charge according to collaboration status*.
The cost recovery guide for 2022 is:
- FFPE tissue $250 (extra processing fees may apply)
- Fresh tissue $315
- Blood aliquot $20
- Clinical Data $50 per hour
- QOL Data $50 per hour
- MBS/PBS data please contact MRV regarding additional requirements
- Administration to be determined on a case-by-case basis
- Courier Researchers cost
*Collaboration status is defined as anticipated authorship from research outputs of an investigator from an MRV participant.
Categories For Collaboration
The following are the categories available for collaboration with MRV:
- MRV participant is defined as an entity is subject to the MRV research collaboration agreement for investigator-initiated projects
- External Academic investigators and entities (with MRV collaboration) include not-for-profit institutions such as Universities, Medical Research Institutes and Hospitals that are not MRV participants where the Principal Investigator for the Project is employed by the academic entity.
- External Academic investigators and entities (without MRV collaboration) include not-for-profit institutions such as Universities, Medical Research Institutes and Hospitals that are not MRV participants where the Principal Investigator for the Project is employed by the academic entity.
- Commercial investigators and groups indicate for-profit entities such as pharmaceutical and biotechnology companies.
Researcher Obligations
Researchers using MRV resources are obliged to formally acknowledge MRV in all related presentations and publications.
The MRV Acknowledgment for Papers and Presentations must read:
The authors wish to thank Melanoma Research Victoria and acknowledge the MRV sites contributing to this work: (select one or more)
- Peter MacCallum Cancer Centre,
- Victorian Melanoma Service, Alfred Health
- Olivia Newton-John Cancer Research Institute, Austin Health
- Skin Health Institute
- Border Medical Oncology Research Unit
OR
If Melanoma Research Victoria is listed as an author the following statement is included in the acknowledgements or supplementary note area –
The MRV Principal Investigators for this project are: (select one or more) Grant McArthur, Victoria Mar, Damien Kee, Peter Foley and Craig Underhill