Genetic testing in melanoma: an interview with Dr. Diane McDowell, US Medical Affairs Lead, Oncology, GSK
Source: News Medical, August 2014

Please can you give a brief history of genetic testing?
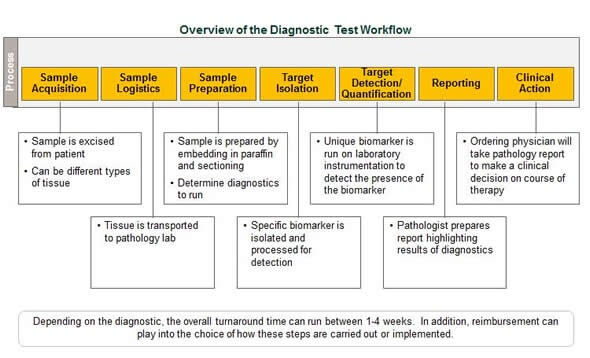
Genetic testing, the commonly used process of looking for changes in a person’s genes or chromosomes that may be associated with specific diseases or conditionsi, dates back to before President Richard Nixon declared the War on Cancer with the passage of the National Cancer Act in 1971.ii
The earliest genetic tests helped detect abnormalities in single genes causing rare, inherited disorders like cystic fibrosisiii, sickle cell anemia and Tay-Sachs disease.iv By the 1980s, researchers were beginning to better understand the role of genes in cancerv, with the first genetic test for breast and ovarian cancer being introduced in the mid-1990s.vi
In recent years, the variety of tests has greatly expanded.ii With today’s genetic tests, healthcare professionals may determine a person’s risk of developing certain diseases, confirm a suspected diagnosis, detect when an individual might pass a genetic mutation to his or her children, or screen out patients who are unlikely to respond to certain therapiesi for diseases like melanoma.
Which conditions is genetic testing currently available for?
Today, genetic testing is available for over 2,000 rare and common conditions.viii While oncology is an area that has received a significant amount of attention and funding for research, experts predict that over the long term, immunology, central nervous system, infectious diseases and cardiovascular disease will be active areas of research in the area of genetic testing and personalized medicine.
Why has the application of genetic testing to melanoma only occurred more recently?
Over the past 20 years, the growth in knowledge of molecular biology and the genetics of cancer has not only enriched our understanding of how tumours grow, but has also taught us that not every tumour is the same. We’ve known for some time that genetic mutations can affect how cells grow and divide. Certain mutations can cause cells to grow out of control, which may lead to cancer.i
In order to apply genetic testing to melanoma, it was necessary to first gain an understanding of the genetic mutations associated with this particular disease. 2002 signaled a turning point for Stage IV melanoma, when “Mutations in the BRAF Gene in Human Cancer” was published in the journal Nature.viii
The research, funded by the Wellcome Trust Sanger Institute’s Cancer Genome Project, investigated the mitogen-activated protein kinase (MAPK) pathway and described the somatic activating mutations of the BRAF protein in melanomaix, which enabled cancer cells to grow and spread. Thanks to this early research, today we can genetically differentiate at least three sub-types of tumour associated with melanoma, the most common being those with BRAF V600 mutations.
Can the current genetic tests for melanoma determine both types of mutations – inherited and acquired?
Cancers like melanoma can have either inherited (germline) mutations or those that are somatic, or acquired through environmental factors; still other mutations may have no identifiable cause.x
Research has identified some high-risk susceptibility genes thought to be associated with melanoma, including CDKN2A, CDK4, and BRCA1-associated protein (BAP-1).xi While testing is available to determine germline mutations in CDKN2A,xii such mutations are rarexiii and there is currently no clear clinical utility for this test.
There are two key molecular signaling pathways that may somatically contribute to the development of melanoma: the mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K) pathways.xiv As mentioned above, the MAPK pathway, which is dysregulated in many melanomas, contains genes like BRAF and NRAS that can become somatically mutated. xv
Approximately half of all people with metastatic melanoma have a BRAF V600 mutation. BRAF V600E and V600K mutations account for approximately 70?percent and 23?percent, respectively, of patients with a V600 mutation,xvi and can be detected in a tumour via genetic testing. One such FDA-approved test is the THxID® – BRAF, for which GSK collaborated with the in-vitro diagnostic company bioMérieux.

![]()
What percentage of melanoma cases are caused by acquired mutations linked to sun exposure?
Over 90 percent of melanoma cases have been linked to sun exposure.xvii Only approximately 10 percent of melanoma cases may be attributed to germline genetic mutations that are passed within a family from generation to generation.xviii, xix
At what stage are genetic tests for melanoma typically carried out?
The National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology for Melanoma recommend genetic testing be performed at the diagnosis of metastatic melanoma.
If a healthcare provider is considering treating a patient who has metastatic melanoma with a targeted therapy, they should be tested to determine whether their tumour demonstrates a gene mutation.
Once a patient’s melanoma mutation has been identified, they may find they are a candidate for therapy targeted to that particular mutation. xx
What impact can the results of genetic testing have?
Patients who receive a positive diagnosis for a specific mutation within their melanoma tumour can work with their doctor to identify specific targeted therapies that may be available and decide on a treatment course that is best for them.
An improved understanding of the molecular basis of melanoma has presented great opportunity for new and more targeted approaches to treatment, reducing the number of patients who may not derive real benefit for a therapy.
Combinations of targeted therapies, like the combination of Tafinlar® (dabrafenib) and Mekinist® (trametinib) that was approved by the FDA in January 2014, will be an important part of the personalized medicine strategy that stems from genetic testing.
What do you think the future holds for genetic testing in melanoma and how do GSK plan to add to this?
Our belief is that as science and technology continue to evolve, the future of genetic testing will expand beyond a single test for a single mutation toward a multi-plexed approach of evaluating large numbers of genes simultaneously.
One of the biggest breakthroughs in terms of technology is the decrease in turnaround time and the cost of sequencing, which may eventually help identify additional genes of interest to inform personalized treatment for patients.
Genetic testing will also have an increasingly important role in pharmaceutical R&D, as well; a 2013 analysis by McKinsey found that presently, nearly half of the preclinical and Phase I assets in the pharma pipeline have associated diagnostics, especially in oncology, immunology and CNS. Oncology is one of the most active areas of research for genetic testing and personalized medicine. xxi
When we brought Tafinlar® (dabrafenib) and Mekinist® (trametinib) to market, we took a very scientifically-driven approach and combined the agents in Phase I, prior to receiving confirmatory Phase II data. We are now seeing this trend continue, with combination programs that are starting much earlier in Phase I.
At GSK, we are designing studies with very specific patient populations in mind that have the highest chance of getting some kind of therapeutic benefit. At the heart of this is ensuring we can effectively identify these patients using the right kind of diagnostic.
About Dr. Diane McDowell

Source: News Medical
Diane McDowell, MD was a Howard Hughes Scholar at Pennsylvania State University where she earned a Bachelor of Science degree in microbiology. She also received a medical degree from the Temple University School of Medicine.
After medical school, Diane completed a general surgery residency at Tulane University School of Medicine and a breast surgical oncology fellowship at Fox Chase Cancer Center.
Following a year in private practice as a general and breast cancer surgeon, Diane moved to Bristol-Myers Squibb where she worked as a Director in U.S. Medical Affairs focusing on breast and hepatic cancer.
She served in BMS’ European headquarters as the interim Disease Area Head for Oncology in the EU Markets focusing on melanoma and CML before moving on to global medical affairs where she focused on lung cancer.
Prior to joining GSK, Diane was a Director of Global Clinical Research at Bristol-Myers Squibb and was responsible for melanoma and lung cancer trials.
Currently, Diane is the US Medical Affairs Lead for both Tafinlar® (dabrafenib) and Mekinist® (trametinib) for GSK.
Originally from Pittsburgh, Pennsylvania, Diane now lives in Philadelphia with her husband and son.
